Clinical Evidence
Three Phase III clinical trials show superior pain relief lasting through 6 months compared to trial data from other OA pain management injections.1,3,4
In a major clinical trial Cingal 13-01, Cingal® demonstrated statistically significant effectiveness in providing rapid and long-term relief of the pain and symptoms associated with knee osteoarthritis.1
CLINICAL STUDY DESIGN
- Randomized, double-blind, multi-center, placebo-controlled Phase 3 study with an additional active hyaluronic acid (HA) alone comparator arm
- 368 subjects were enrolled in the study with knee osteoarthritis Kellgren-Lawrence grades I, II, or III
- Time points measured: 1, 3, 6, 12, 18, and 26 weeks
- The primary endpoint was the change from baseline in knee pain as measured by the WOMAC Pain Score (100 mm VAS) through 12 weeks post treatment comparing the Cingal group to the saline control group
STATISTICALLY SIGNIFICANT RESULTS
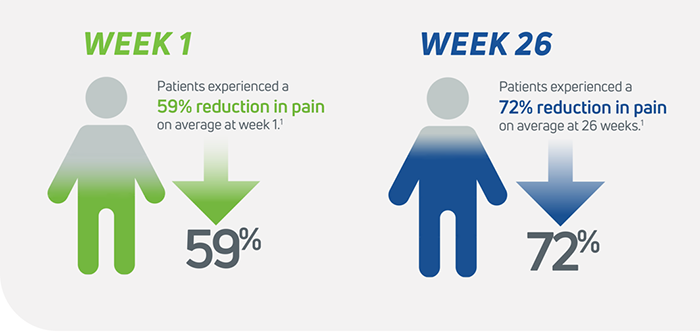
- Cingal delivers faster knee pain relief compared to HA alone, providing significant reduction in pain at Weeks 1 and 31
- Cingal patients experienced long lasting knee pain relief, demonstrating statistically significant reduction in pain through Week 26 relative to saline1
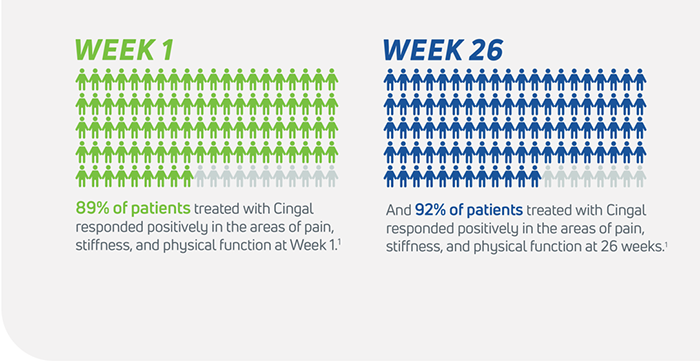
- Cingal is statistically superior to saline at every time point for measurements including pain, stiffness, physical function, and global assessment1
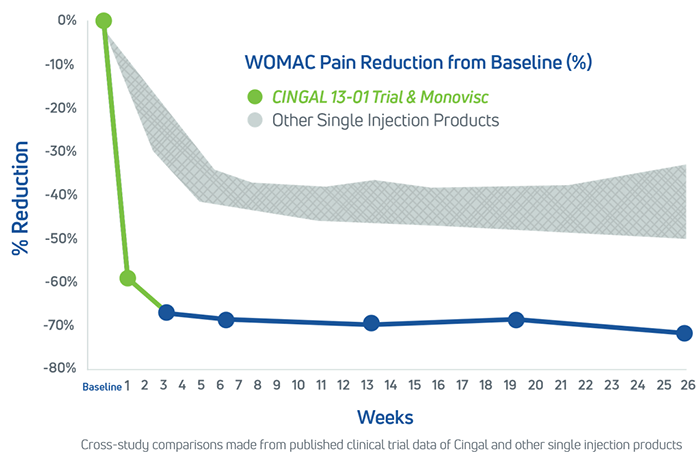
Cingal is statistically superior to saline at 26 weeks in WOMAC pain score delivering a 72% (-42.4mm) improvement relative to baseline.1
POWERFUL PAIN REDUCTION
UNRIVALLED RESPONDER RATES
SAFETY
Cingal demonstrated a very strong safety profile in the study. There were no Serious Adverse Events (SAEs) related to the Clinical Trial Material (CTM). There were a total of six adverse events (AEs) considered related to the CTM; three affecting Cingal subjects. The six events (arthralgia, rash, or peripheral edema) are common and transitory side effects seen with viscosupplements and were resolved without sequelae.
STUDY CONCLUSION
“The pivotal study results unequivocally demonstrate the strong clinical benefit of Cingal”, said Global Principal Investigator Prof. Laszlo Hangody, MD, Ph.D., DSc.
“Patients receiving Cingal experienced significant symptom relief from the first week that continued and even strengthened throughout six months.”
Cingal was determined to be decisively superior to the saline control, delivering a 72% improvement (-42.4 mm) in WOMAC Pain Score at 26 weeks relative to baseline. Cingal met the primary endpoint and all secondary endpoints relative to saline, demonstrating strong statistically significant superiority over saline for the following measures:
- WOMAC Pain Score through 12 weeks and 26 weeks
- Patient Global Assessment through 12 weeks and 26 weeks
- Evaluator Global Assessment through 12 weeks and 26 weeks
- OMERACT- OARSI Responders through 12 weeks and 26 weeks
- WOMAC Stiffness Score through 26 weeks
- WOMAC Physical Function Score through 26 weeks
- Total WOMAC Score through 26 weeks
Cingal also verified its product design objective, demonstrating that an ancillary corticosteroid provides significant early pain relief additive to the long-term pain relief provided by the HA viscosupplement.
Cingal showed superiority over a leading single-injection HA viscosupplement at Week One and Week Three, demonstrating early pain relief provided by the corticosteroid.
The results of the Cingal 13-01 study confirm the safety and effectiveness of a single intra-articular injection of Cingal for rapid and long-term relief of joint pain and symptoms in patients with OA of the knee.
RETREATMENT STUDY RESULTS
The safety of a repeat injection of Cingal was confirmed in the Cingal 13-02 study.
In the Cingal 13-02 study, 94 patients who had received Cingal initially received an open-label injection of Cingal and were monitored for adverse events (AEs). The retreatment study’s key findings were:
- A low number of subjects (4.3%) experienced an adverse event (AE) related to the study injection. Observed AEs were typical of those associated with viscosupplements (arthralgia, injection site pain, swelling, and erythema), and over 95% were considered ‘mild’ or ‘moderate’ in severity. All AEs were transitory, resolving without treatment.2
- The AE rate associated with Cingal was found to be consistent across both first-time and repeat injection studies. There were no statistically significant differences between the AE profile of participants in the Cingal 13-01 study (single injection) and those in the Cingal 13-02 study (repeat injection).2
The results of this secondary study combined with the initial Phase 3 data show that Cingal maintains a consistently strong safety profile in both an initial injection as well as a repeat injection.
1. Hangody L, et al Intraarticular Injection of a Cross-Linked Sodium Hyaluronate Combined with Triamcinolone Hexacetonide (Cingal) to Provide Symptomatic Relief of Osteoarthritis of the Knee: A Randomized, Double-Blind, Placebo-Controlled Multicenter Clinical Trial. Cartilage. 2018 Jul;9(3):276-283. doi: 10.1177/1947603517703732. Epub 2017 May 23. PMID: 28535076; PMCID: PMC6042027.
2. Data on File (13-02)
3. Data on File (16-02)
4. Data on File (19-01)