Clinical Overview
The results from the Cingal® clinical study showed statistically significant rapid and long lasting knee pain relief in patients with early to moderate osteoarthritis (OA).

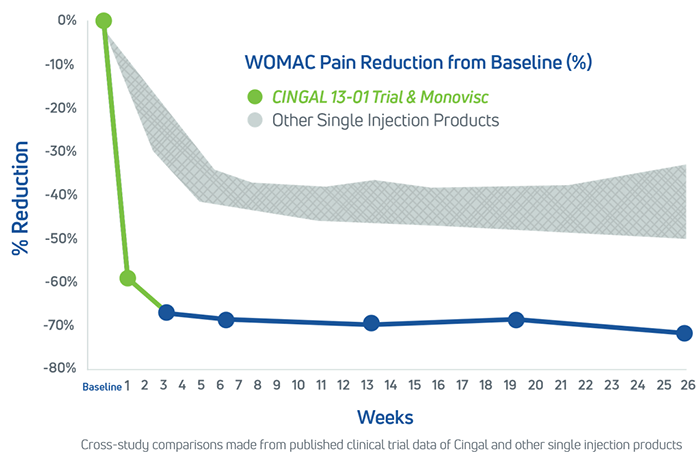
With one single injection treatment, Cingal is clinically proven to deliver pain relief within days that lasts for 6 months.1
In its clinical trial, Cingal was compared to single injections of a leading single-injection HA viscosupplement and saline (normal salt solution: control) with time point measurements at weeks 1, 3, 6, 12, 18 and 26. There were 368 patients enrolled in the study with knee osteoarthritis.1
IS IT CLINICALLY EFFECTIVE?
- Cingal delivers faster pain relief compared to a leading single-injection HA viscosupplement, providing statistically significant reduction in pain at Weeks 1 and 3 relative to HA viscosupplement and saline1
- Cingal patients experienced long lasting pain relief, demonstrating statistically significant reduction in pain through Week 26 relative to saline1
IS IT CLINICALLY SAFE?
Cingal demonstrated a very strong safety profile in its clinical study.
- There were no serious adverse events related to the study device
- There were only six adverse events related to the study device including knee joint pain, rash and swelling, which are common and minor side effects seen with viscosupplements and were all resolved naturally.
IS RETREATMENT CLINICALLY SAFE?
The safety of a repeat injection of Cingal was confirmed in a secondary clinical study. Participants from the primary Cingal clinical study who had received an initial injection of Cingal received a repeat injection after 6 months. A second injection of Cingal was well tolerated by patients who received a repeat injection in the secondary study.
Over 95% of the patients who received a repeat injection of Cingal tolerated it without any problems related to Cingal. The low number of patients who had adverse events only experienced minor side effects typical of those associated with viscosupplements (knee joint pain, injection site pain, swelling, redness, or rash), and all of them were resolved naturally without treatment.2
STUDY CONCLUSIONS
- In its primary study, Cingal was determined to be decisively and statistically superior to saline at every time point for measurements including pain, stiffness, physical function, and global assessment1
- Cingal demonstrated that an ancillary corticosteroid provides a significant early pain relief additive to the long-term pain relief provided by a leading HA viscosupplement1
- The effectiveness of a single intra-articular injection of Cingal for rapid and long-term relief of knee joint pain due to knee osteoarthritis was confirmed in its primary clinical study1
- The results of the primary and secondary Cingal clinical studies show that Cingal maintains a consistently strong safety profile in both an initial injection as well as a repeat injection1,2
First in its class, Cingal delivers clinically proven safety and efficacy to provide rapid and long-term knee pain relief provided by the combination of a fast acting corticosteroid with a long lasting HA viscosupplement.
1. Cingal 13-01, a randomized, double-blind, placebo-controlled, active comparator Phase III study. Anika Therapeutics, Inc.: study sponsor, Dr. Laszlo Hangody: global principal investigator, SynteractHCR: CRO.
2. Cingal 13-02, an open-label, follow-on study to Cingal 13-01. Anika Therapeutics, Inc.: study sponsor, Dr. Laszlo Hangody: global principal investigator, SynteractHCR: CRO.